Retinal diseases: RNA therapeutics show promise but is India ready? Premium
Retinal diseases: RNA therapeutics show promise but is India ready? Premium
Vision is crucial to navigate the world, connect with others, and perform everyday tasks. It helps us perceive colours, shapes, and movement, which are essential to learn, work, and keep safe.
According to the World Health Organisation, more than 2.2 billion people worldwide experience some form of vision impairment. The causes range from cataracts and diabetic retinopathy to glaucoma, age-related macular degeneration, and inherited retinal diseases (IRDs).
IRDs are genetic conditions that lead to progressive vision loss, often resulting in blindness. These diseases stem from mutations in more than 300 genes responsible for the function of the retina, the light-sensitive tissue at the back of the eye. While some individuals may lose their sight shortly after birth, others experience gradual deterioration over time. In many cases, early intervention could slow down or even prevent the progression of blindness.
An estimated 5.5 million people suffer from IRDs around the world, with a prevalence rate of one in 3,450. However, the situation is more critical in India. Studies have revealed significantly higher prevalence, with one in 372 individuals in rural South India,one in 930 in urban South India, and one in 750 in rural Central India affected by these conditions.
In 2017, the U.S. Food and Drug Administration (FDA) made a historic move by approving the first gene therapy for blindness caused by mutations in the RPE65 gene. This approval sparked hope for patients with other genetic causes of blindness. Currently, more than 50 clinical trials are exploring gene therapy as an option to treat various inherited eye disorders.
In India, however, awareness among clinicians about the availability and potential of RPE65 gene therapy remains limited. While gene therapy has proven revolutionary, it is not yet a universal solution for all genetic eye diseases. This is where RNA-based therapies are poised to make a significant impact.
RNA-based precision therapeutics are emerging as a game-changer for genetic disorders, including IRDs. Unlike DNA or genome-editing therapies, RNA-based therapies offer a safer alternative as they make temporary changes that don’t carry over to future generations, reducing the risk of unintended long-term effects.
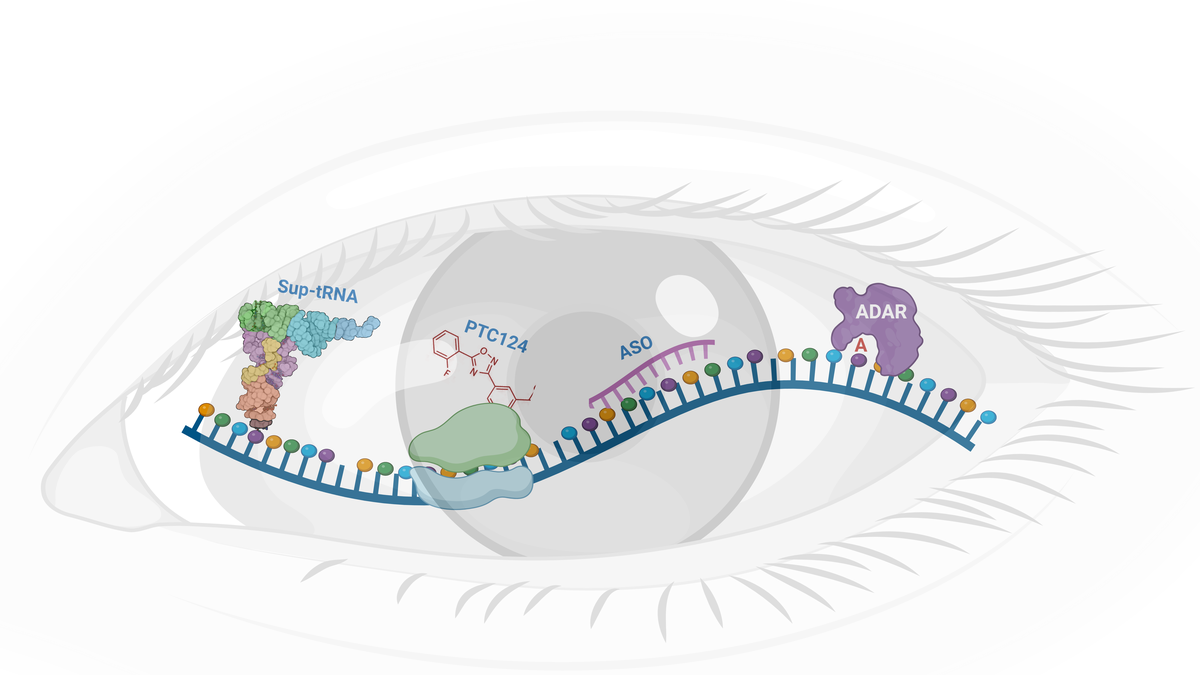
Recent advancements have introduced RNA-based therapies like antisense oligonucleotides (ASOs), which have already been used successfully to treat diseases such as spinal muscular atrophy and Duchenne muscular dystrophy. Medical researchers are now exploring ASO therapy for retinal conditions like Stargardt disease, Leber congenital amaurosis, and retinitis pigmentosa.
Beyond ASOs, researchers are also developing more advanced RNA-based options to address IRDs. One promising approach involves RNA-editing with ADAR enzymes, which can correct specific genetic mutations at the RNA level. This method has the potential to restore protein production in retinal cells without altering the underlying DNA, offering a new way to treat retinal degenerative diseases caused by single-point mutations.
Another innovative strategy is the use of suppressor tRNAs to bypass stop-codon mutations, which can prematurely halt protein synthesis in retinal cells. By enabling the production of full-length proteins, this approach could help restore proper retinal function in IRD patients, where stop-codon mutations disrupt vital protein production.
Another potential small molecule RNA-based therapy is PTC124, also known as ataluren, which is already being used to treat patients with cystic fibrosis and Duchenne muscular dystrophy. Recently, clinical trials have begun to investigate its use in treating a rare developmental eye disease called aniridia.
Taken together, these options offer a more targeted, personalised treatment approach that could halt the progression of IRDs and improve patient outcomes with greater precision.
Precision medicine is an approach that tailors treatments to an individual’s genetic makeup, lifestyle, and other factors, offering a more targeted alternative to the one-size-fits-all approach of traditional options.
For rare diseases like IRDs, understanding the genetic mutations prevalent in a population is essential for researchers to develop effective RNA-based therapies. Although researchers have linked more than 300 genes to IRDs, research in India has yet to fully map the genetic mutations responsible for these conditions in the local population.
In fact, there is currently no large cohort study in India (i.e. involving at least 500 patients) to describe the mutation spectrum of IRDs. Such extensive studies are vital for researchers to identify the most common genetic defects that can subsequently be targeted using precision medicine.
For example, the ABCA4 gene is commonly mutated in IRD patients worldwide and is a popular therapeutic target. However, we lack a clear understanding of whether it is just as prevalent in Indian populations and/or whether some other mutation is expressed more often in certain ethnic groups.
India’s large size and diverse population add another layer to this challenge. Genetic mutations can vary significantly across different communities, making it difficult to identify common mutations. Accurately mapping these mutations necessitates extensive, resource-intensive research across various subgroups.
Additionally, there are several barriers, including a lack of awareness of the genetic basis of IRDs among the people at large and healthcare providers alike, limited availability of genetic counselling services, insufficient research funding, and restricted access to diagnostic infrastructure in rural areas.
Thus, to fully unlock the potential of RNA-based therapeutics, India must prioritise genetic research with a particular emphasis on understanding the mutation profiles of people with IRDs, in collaboration with local research institutions and healthcare providers.
A notable example of such a collaboration is a June 2024 study by researchers from the CSIR-Institute of Genomics and Integrative Biology, New Delhi, and the L.V. Prasad Eye Institute, Hyderabad. The teams’ findings led to the development of a precision therapy for a specific form of IRD.
Expanding partnerships between global and local pharmaceutical companies, as well as research institutes, will also make these treatments more accessible to Indian patients. Raising awareness among clinicians and researchers about advances in RNA therapies will likewise be crucial to ensure they are implemented effectively.
Sandeep Sharma Asodu is a postdoctoral fellow at Hadassah Medical School, The Hebrew University of Jerusalem.